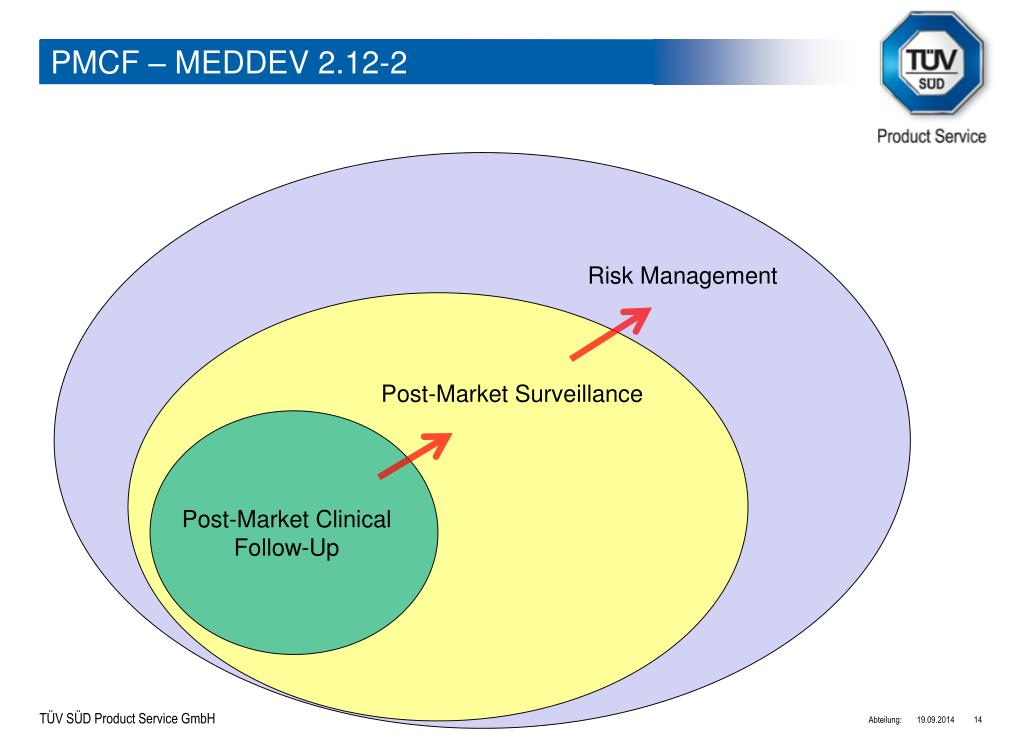
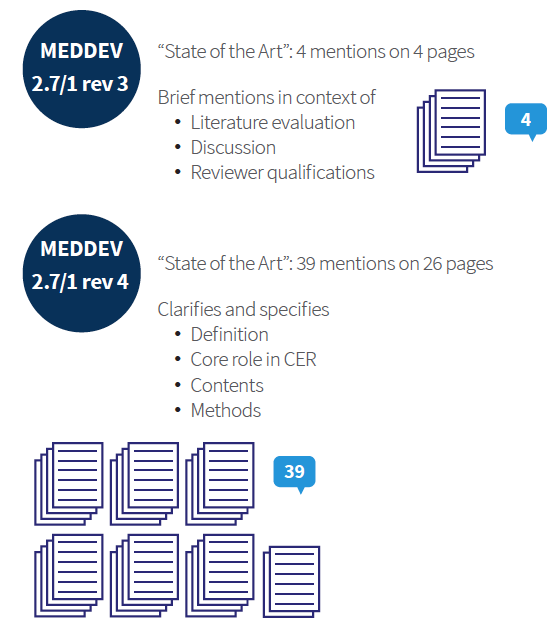
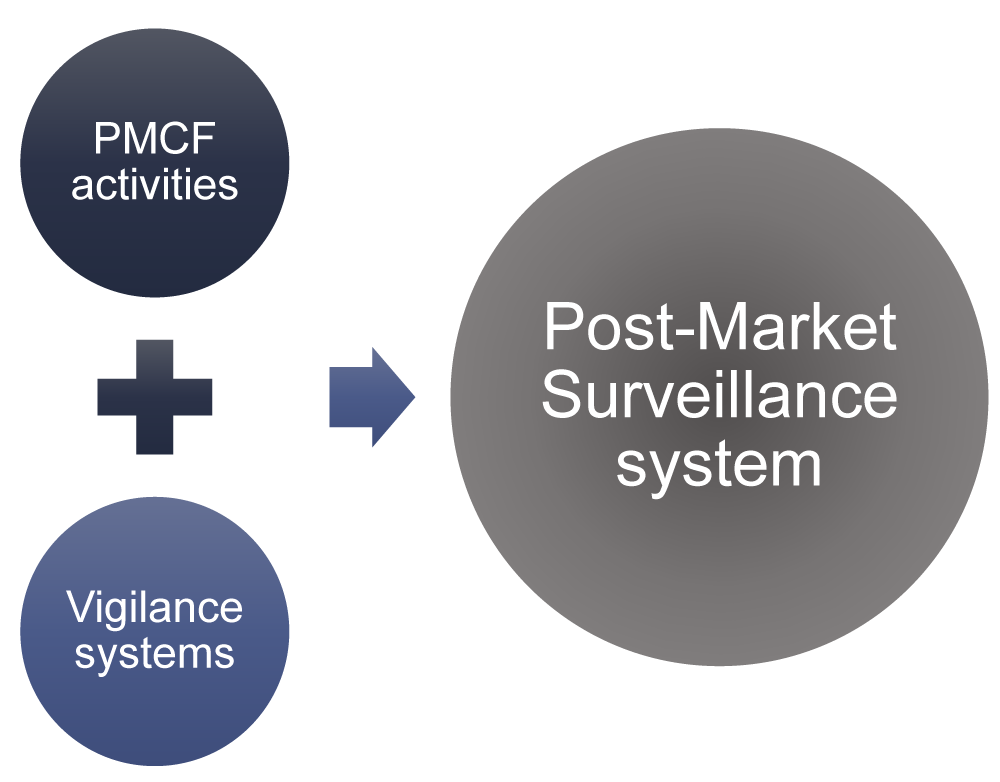
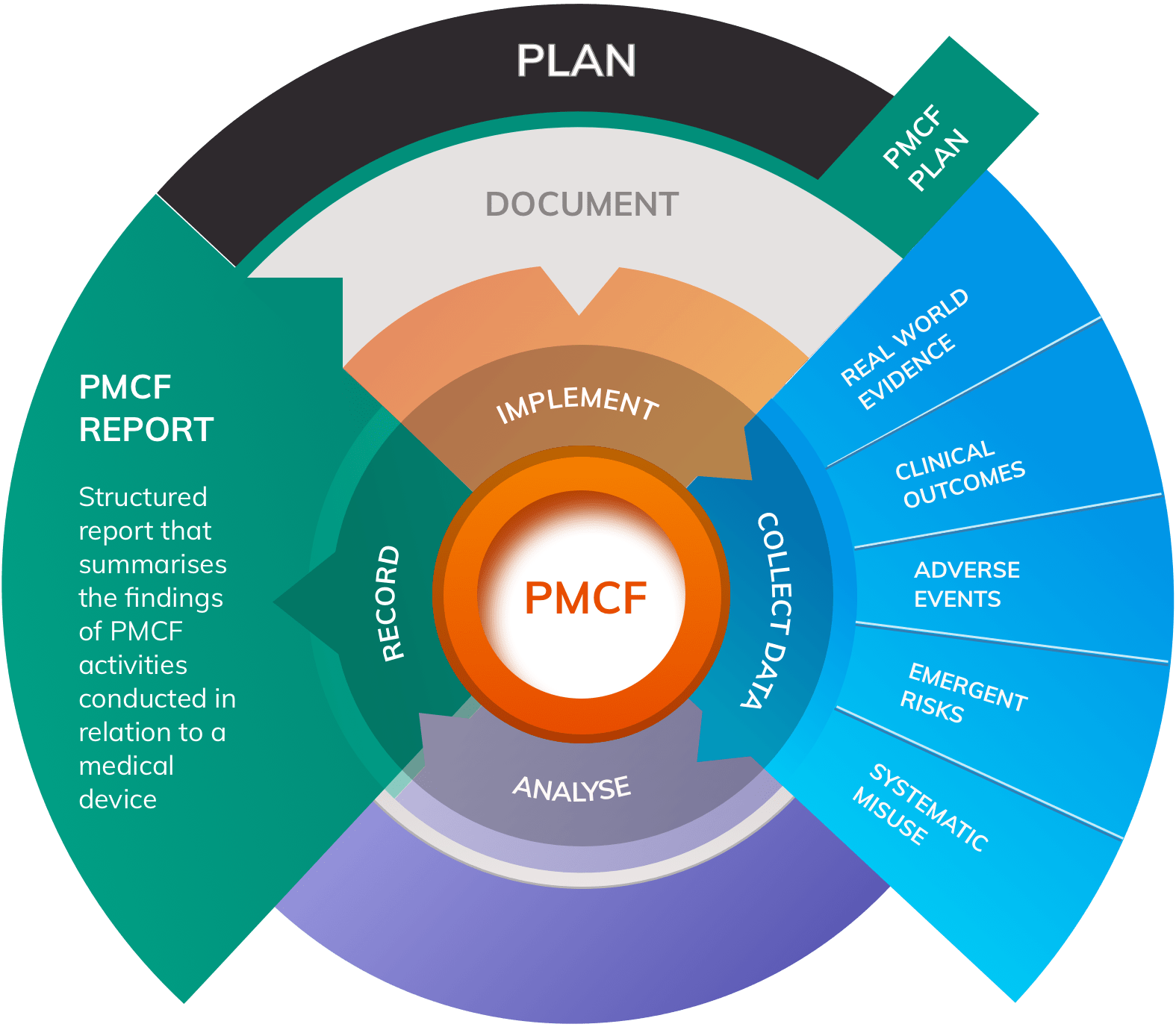
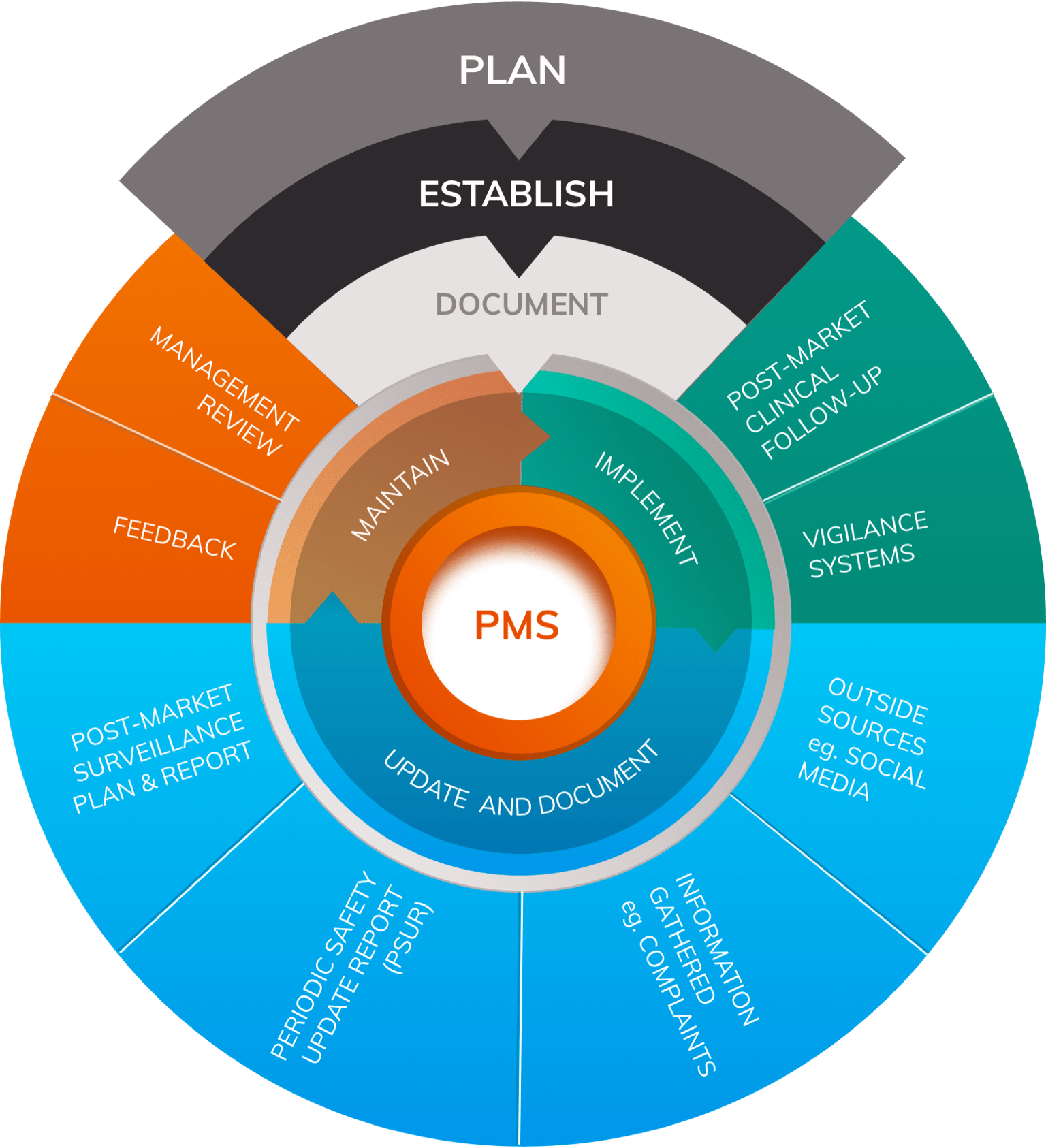
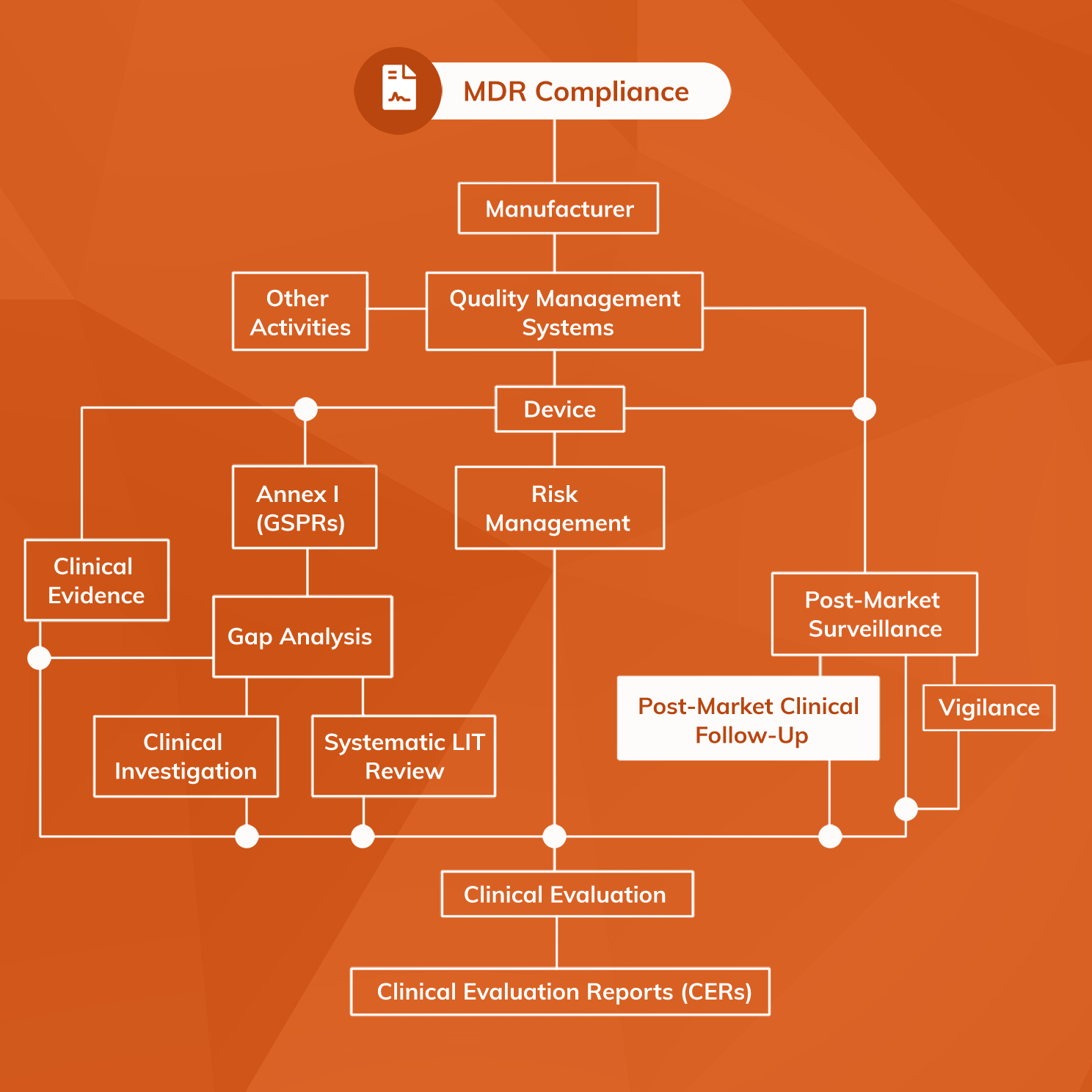
The Post-Market Clinical Follow-up (PMCF) requirements under the European Medical Device Regulation: Step by Step PMCF - GMED Medical Device Certification
Clinical Evaluation Report for Europe and MEDDEV Expectations: Impact with New Medical Device Regulations (MDR) | Medical Events

The Post-Market Imperative: Understanding the requirements for effective post-market clinical follow-up - BONEZONE