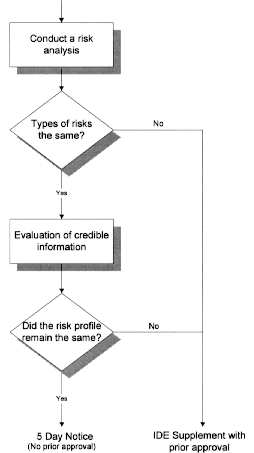
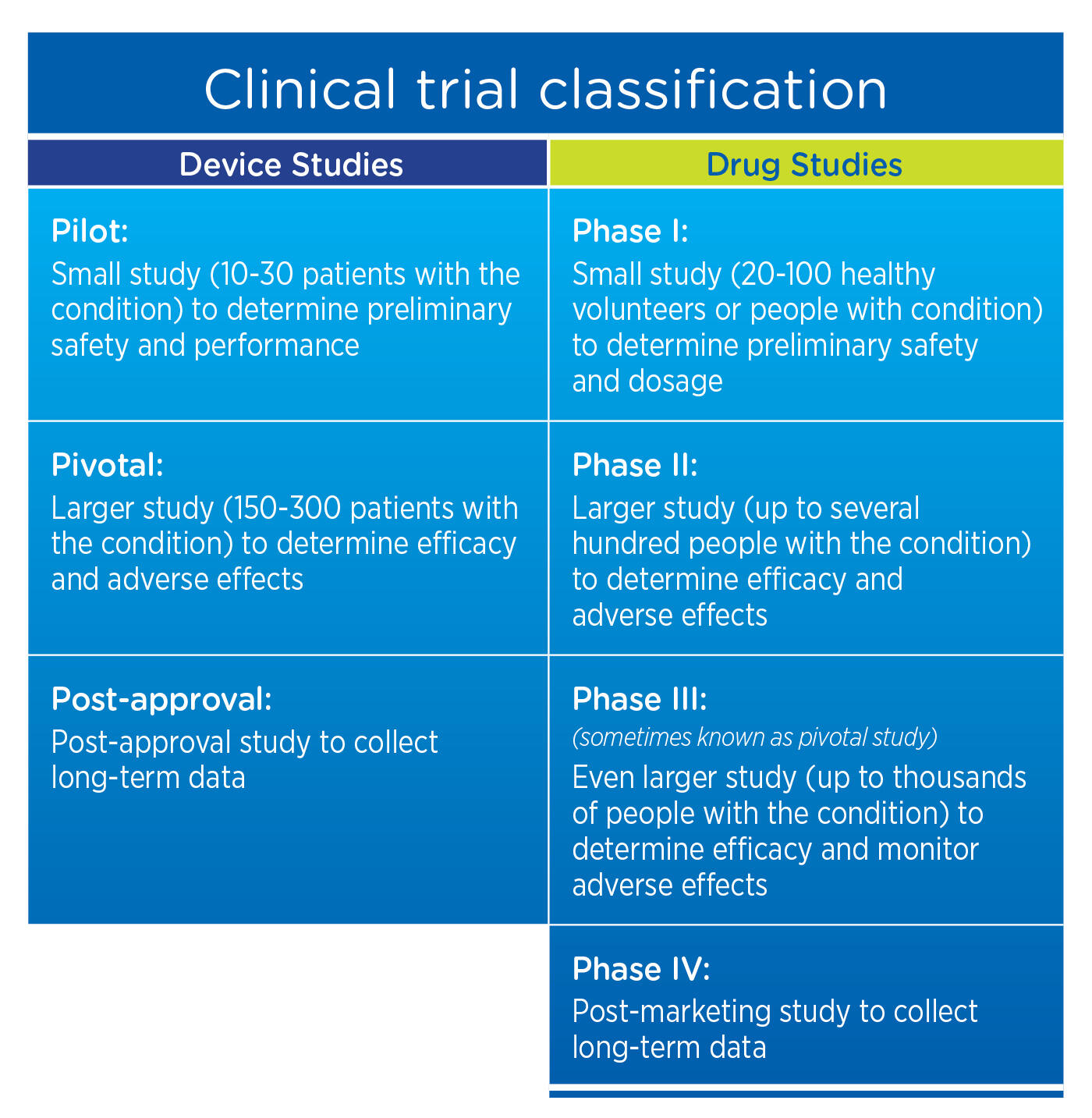
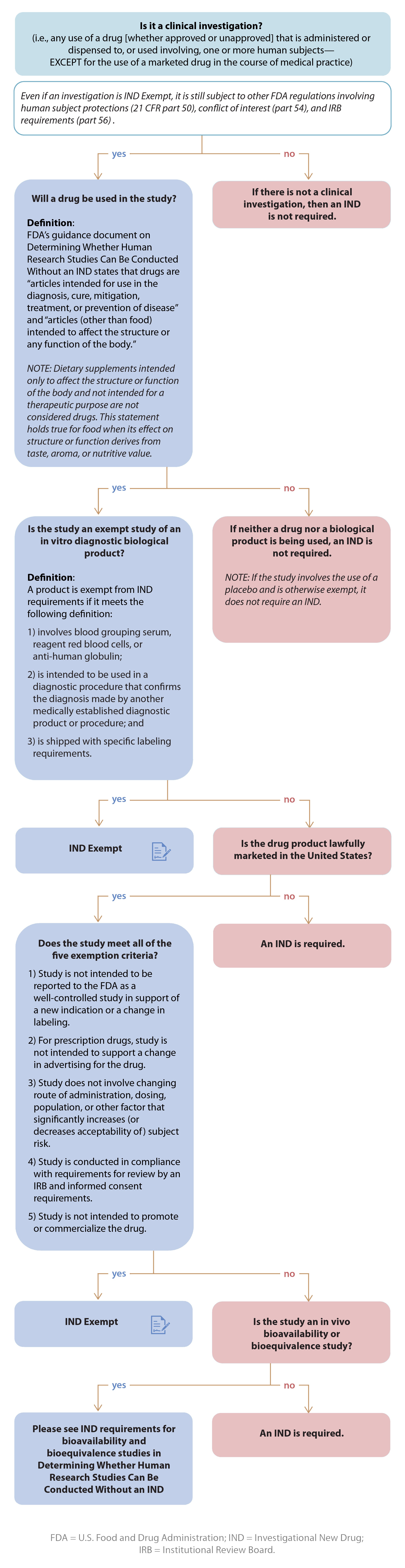
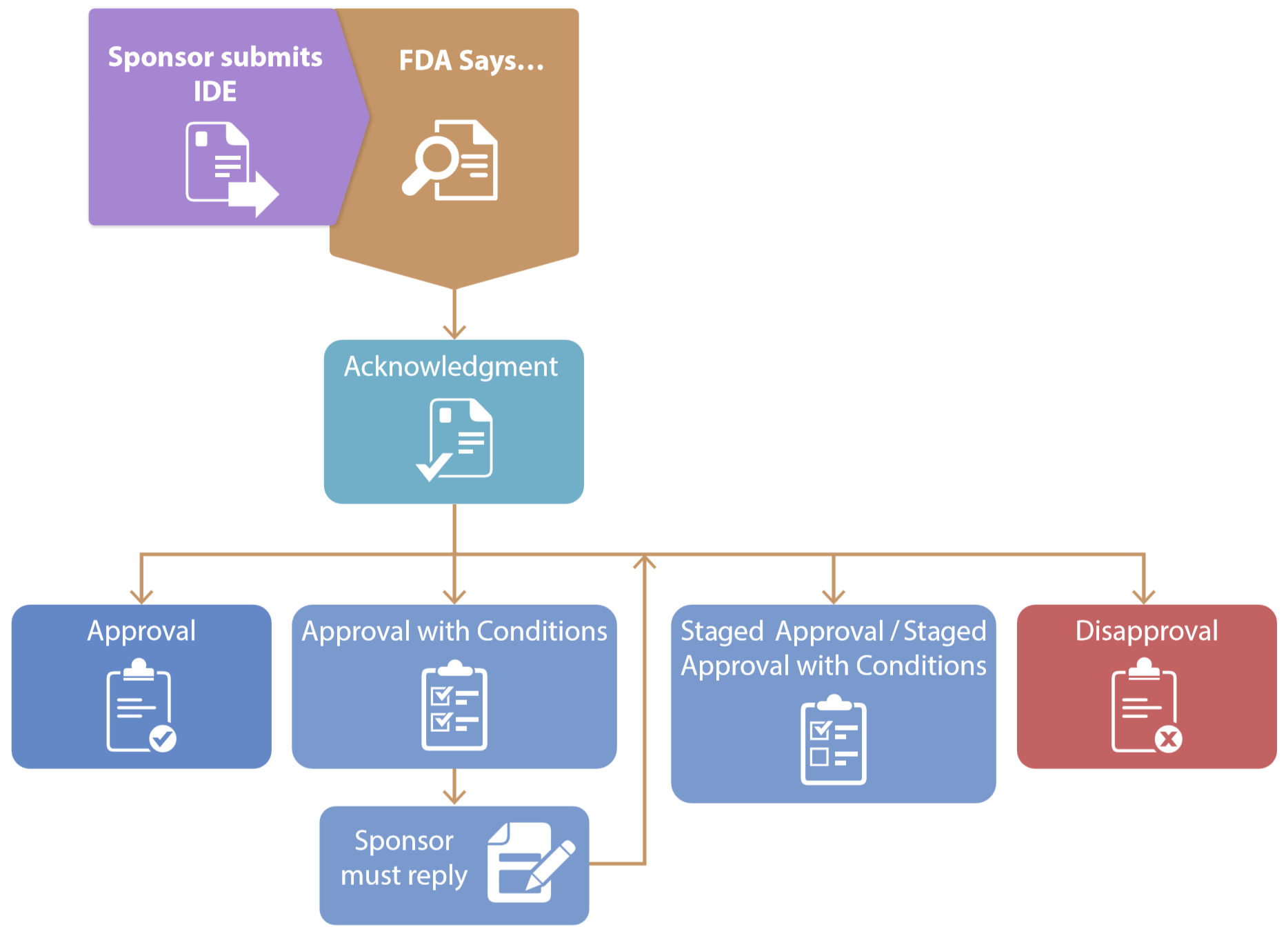
IDE DECISION WORKSHEET For Investigator-Initiated Clinical Investigations Does Your Study Require an IDE Submittal to the FDA? N
FDA Decisions for Investigational Device Exemption Clinical Investigations - Guidance for Sponsors, Clinical Investigators, Inst

Updated FDA Guidance Helps Device Study Sponsors Better Anticipate Coverage for Investigational Devices | Advisories | Arnold & Porter
FDA Categorization of Investigational Device Exemption (IDE) Devices to Assist the Centers for Medicare and Medicaid Services (C
FDA Categorization of Investigational Device Exemption (IDE) Devices to Assist the Centers for Medicare and Medicaid Services (C