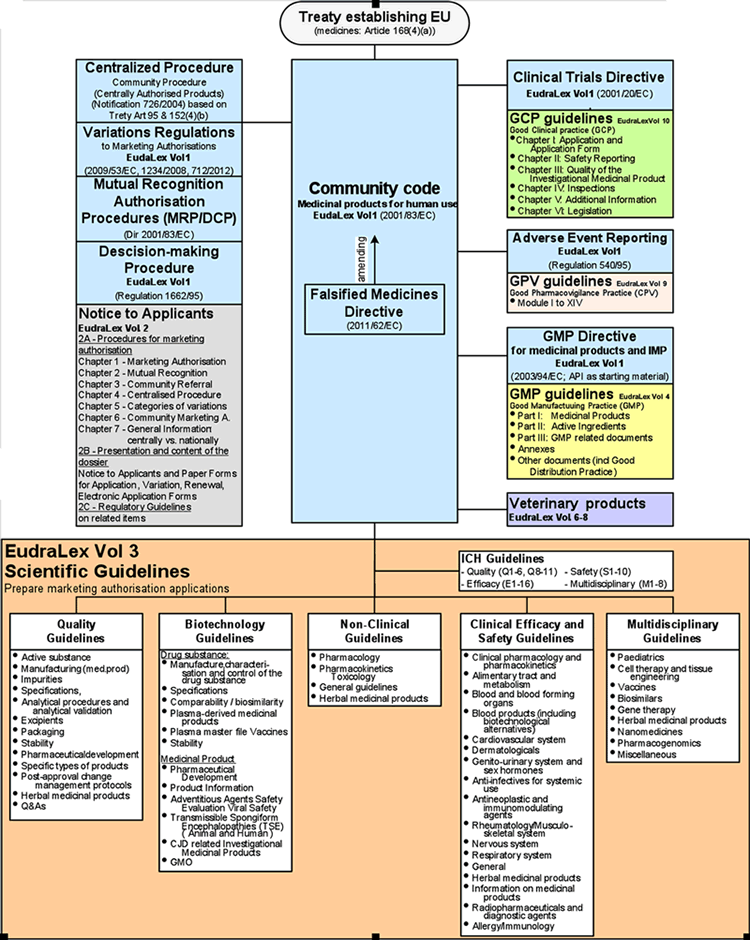
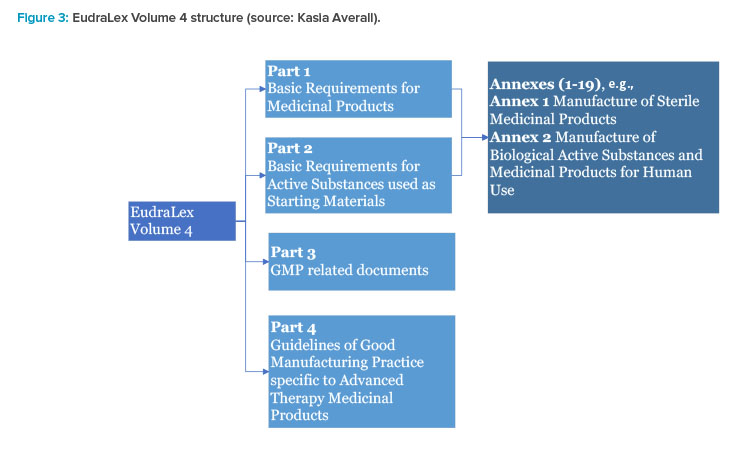
EUROPEAN COMMISSION EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manuf

EudraLex - Volume 4 - Good Manufacturing Practice (GMP) guidelines 2017 - Free PDF Download | M A N O X B L O G

PPT - REVISION OF EUDRALEX VOL. 4 - GMP Luisa Stoppa, Ph.D. Inspection and Certification Department PowerPoint Presentation - ID:6720277

PPT - REVISION OF EUDRALEX VOL. 4 - GMP Luisa Stoppa, Ph.D. Inspection and Certification Department PowerPoint Presentation - ID:6720277

The Handbook of Basic GMP Requirements: Collected guidelines from Eudralex Volume 4, Part I “Basic Requirements for Medicinal Products”: Santoro, Karyn Noemi: 9798552183685: Amazon.com: Books

Good Manufacturing Practice (GMP) Guidelines: The Rules Governing Medicinal Products in the European Union, EudraLex Volume 4 Concise Reference: Allport-Settle, Mindy J.: 9780982147603: Amazon.com: Books
EUROPEAN COMMISSION Brussels, EudraLex The Rules Governing Medicinal Products in the European Union EU Guidelines to Good Manu
Concept paper on the revision of annex 4 of the guidelines on good manufacturing practice – manufacture of veterinary medicina

EudraLex - Volume 4 - Good Manufacturing Practice (GMP) guidelines - Free PDF download | M A N O X B L O G