Adverse Event of Special Interest/AESI/AESI in Pharmacovigilance/AESI in CT/Pharmacy job interview - YouTube

Ministry of Health on Twitter: "#Unite2FightCorona #LargestVaccineDrive DCGI approves “Conditional Market Authorization” of two #COVID19 Vaccines- Covaxin and Covishield. https://t.co/kpDLdyYm48 https://t.co/fr5kMQv2OZ" / Twitter

Primary results of STRONG: An open-label, multicenter, phase 3b study of fixed-dose durvalumab monotherapy in previously treated patients with urinary tract carcinoma - European Journal of Cancer

Cumulation of Safety Data from a Randomized Clinical Trial, the AESI is... | Download Scientific Diagram
Guide for Surveillance of Adverse Events of Special Interest (AESI) during novel Oral Polio Vaccine type 2 (nOPV2) Use
Peer review fail: Vaccine publishes antivax propaganda disguised as “reanalyses” of Pfizer and Moderna COVID-19 vaccine clinical trial data | Science-Based Medicine
How to Investigate a Serious Adverse Event Reported During a Clinical Trial for a COVID-19 Vaccine | SpringerLink
Guide for Surveillance of Adverse Events of Special Interest (AESI) during Novel Oral Polio Vaccine Type 2 (nOPV2) Use

Challenges in conducting post-authorisation safety studies (PASS): A vaccine manufacturer's view - ScienceDirect
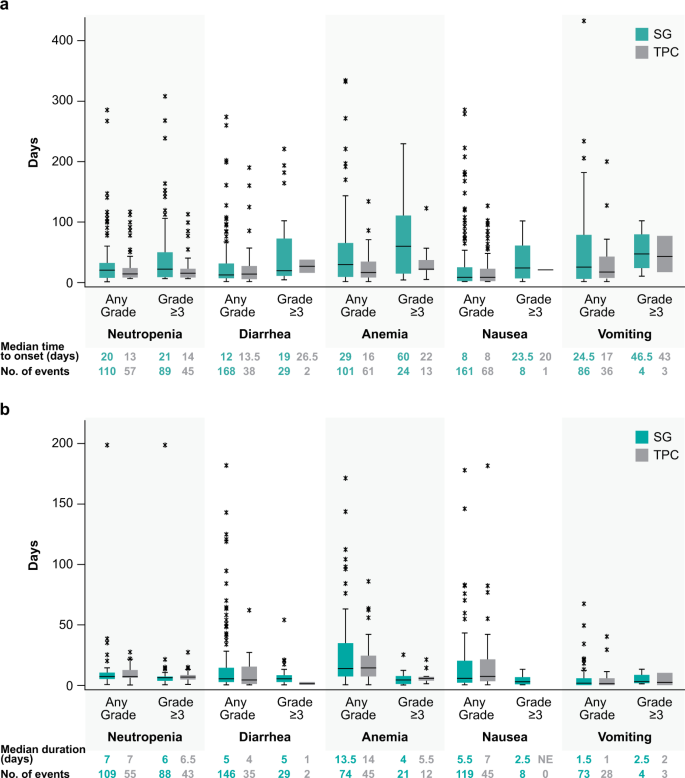
Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer | npj Breast Cancer