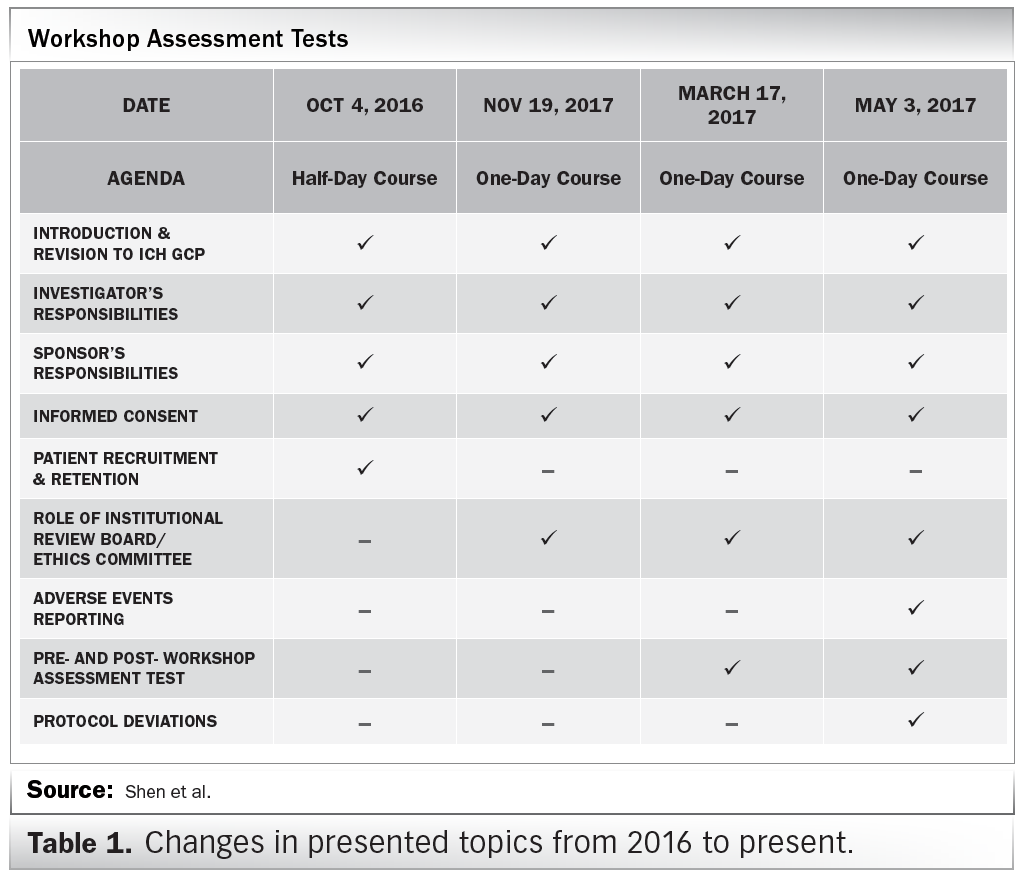
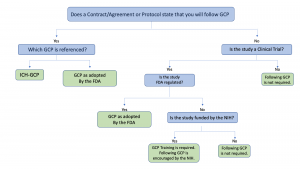
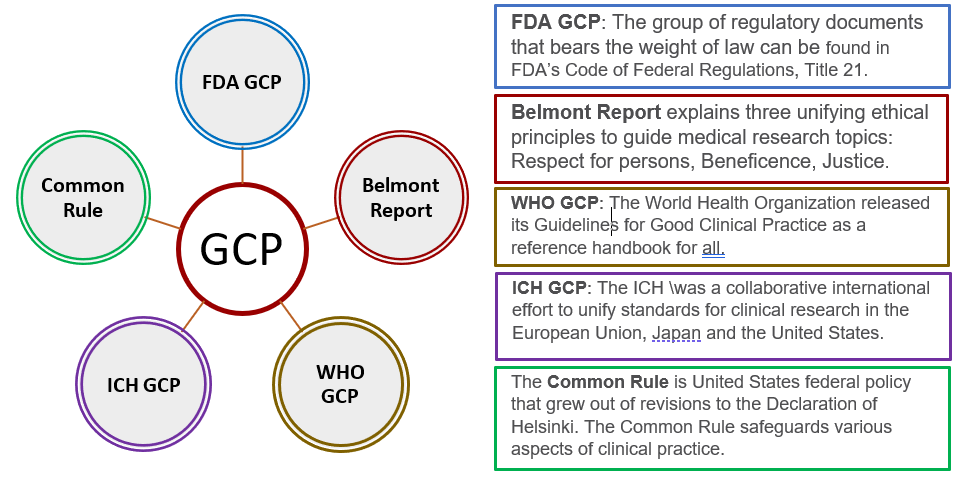
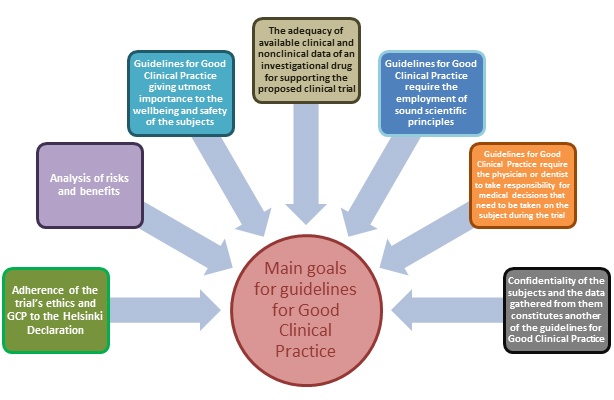
Introduction To Investigators Responsibilities With Good Clinical Practice | PDF | Institutional Review Board | Clinical Trial

REFRESHER: ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Fiona Stanley Hospital) - RETProgram

EU clinical research framework. ICH GCP = International Conference on... | Download Scientific Diagram

What does ICH-GCP mean? - Definition of ICH-GCP - ICH-GCP stands for International Conference on Harmonisation-Good Clinical Practice. By AcronymsAndSlang.com

Consorzio per Valutazioni Biologiche e Farmacologiche - CVBF organises the “ ICH-Good Clinical Practice (GCP) Training Course”, an e-learning training course aimed at providing a guide for all individuals that are involved in

The Good Clinical Practice guideline and its interpretation – perceptions of clinical trial teams in sub‐Saharan Africa - Vischer - 2016 - Tropical Medicine & International Health - Wiley Online Library




.jpg)