The Exclusion of Patients with CKD in Prospectively Registered Interventional Trials for COVID-19—a Rapid Review of International Registry Data | American Society of Nephrology
Prevalence of clinical trial status discrepancies: A cross-sectional study of 10,492 trials registered on both ClinicalTrials.gov and the European Union Clinical Trials Register | PLOS ONE
Clinicaltrialsregister.eu ▷ Observe Clinical Trials Register News | EU Clinical Trials Register - Update

Ongoing Clinical Trials for the Management of the COVID-19 Pandemic: Trends in Pharmacological Sciences

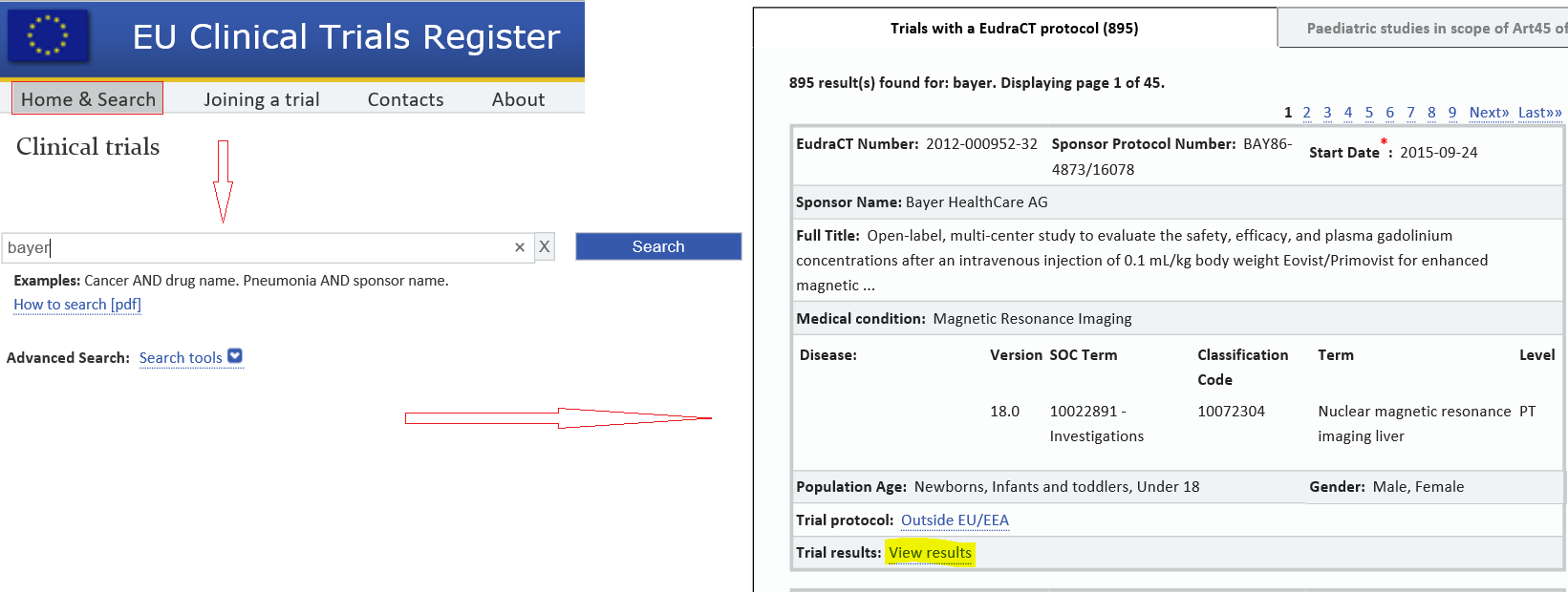
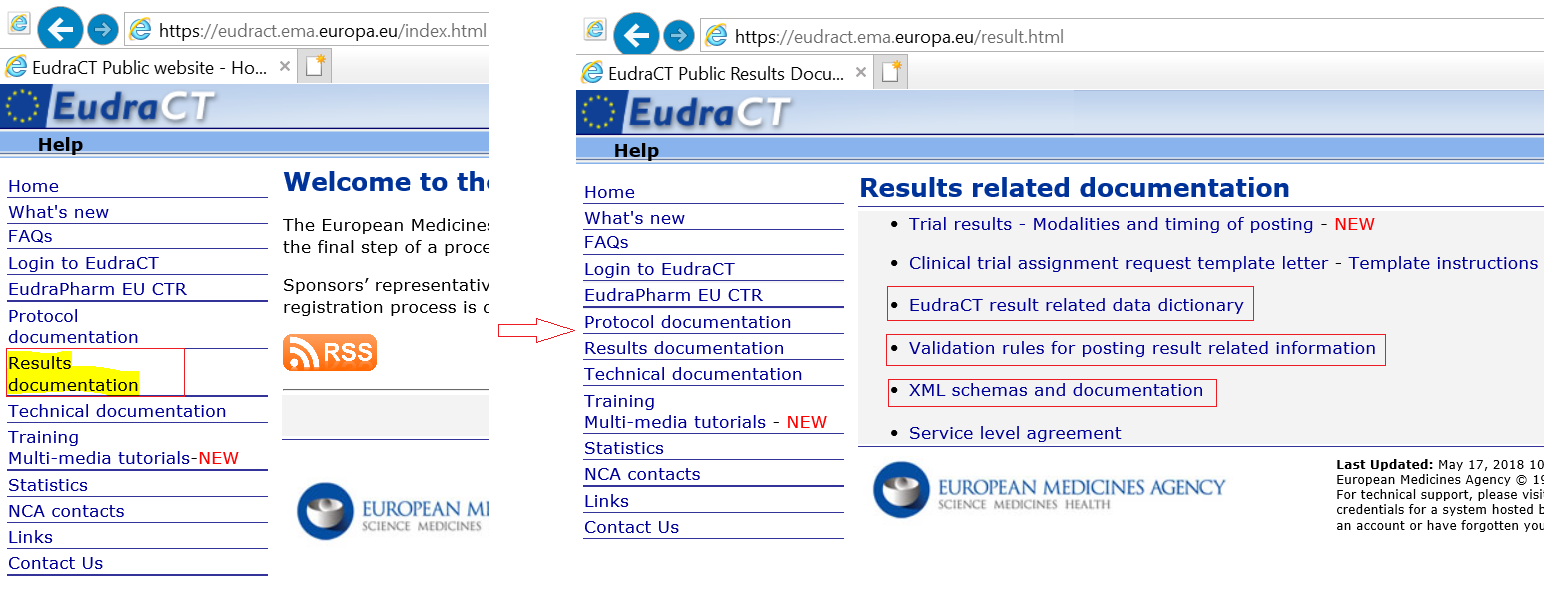
Compliance with requirement to report results on the EU Clinical Trials Register: cohort study and web resource | The BMJ

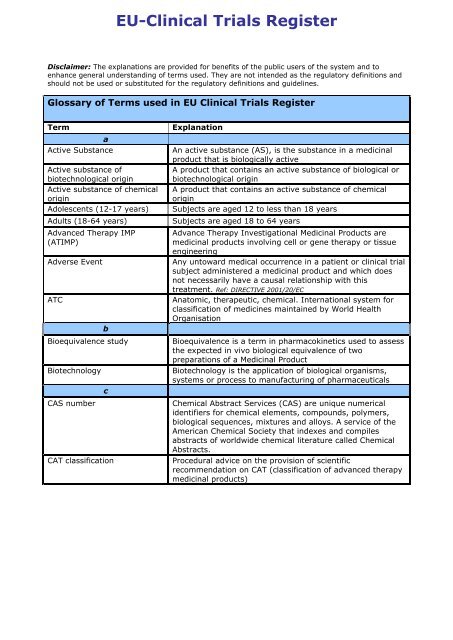
The Current Status of European and National Financial Sources for Clinical Research and Their Impact on Paediatric Non-commercial Clinical Trials: A Case Study of the Czech Republic | SpringerLink